AMOUNT (formerly AMT): dose amount
The AMOUNT column records the amount of the administrated doses. For dose-lines, the value must be a double. For response-lines, the content is free. Note that the interpretation of dose and response lines has changed between the 2016R1 and the 2018R1 versions, as detailed here.
Examples:
- Basic example: the individual 1 receives a dose of 10 at time 0
ID TIME AMT Y 1 0 10 . 1 1 . 6 1 2 . 4
- Example with a single dose split into two routes of absorption: a single dose may be absorbed at different sites, leading to several absorption route. This can be taken into account by splitting the dose into several fractions, directed to several absorption macros. With the following data set:
ID TIME AMT Y 1 0 10 . 1 1 . 6 1 2 . 4
and the following PK block in the model file:
PK:
oral(ka=ka1, p=F)
oral(ka=ka2, Tlag, p=1-F)
a fraction F of the dose is absorbed via a first-ordre process with rate constant ka1, and a fraction 1-F is absorbed with a absorption constant ka2 after a lag time Tlag.
Format restrictions:
- A data set shall not contain more than one column-type AMOUNT.
- For dose-lines with EVENT ID = 1, 3 or 4, the value must be a double.
- For lines without EVENT ID, the value must be a double or a dot ‘.’
ADMINISTRATION ID (formerly ADM): administration identifier
The ADMINISTRATION ID column permits to distinguish different types of administrations, for instance oral administrations and intravenous administrations. The content of the ADMINISTRATION ID column is used for dose-line only and must be a positive integer. This integer works like a flag, which can be used in the model file to link the dose informations of the data set to a specific administration route in the model.
Examples:
- Example with oral and iv administrations: For instance, with the following data set:
ID TIME AMT ADM Y John 0 10 1 . Eric 0 20 2 . Jean 0 10 3 .
and the following PK block in the Mlxtran model file:
PK: iv(adm=1) oral(adm=2, ka)
the subject John will receive a dose of 10 via a bolus iv, while subject Eric will receive a dose of 20 orally with first-order rate constant ka. The identifier in the ADMINISTRATION ID column should match the “adm=” field of the administration macros. A same subject can receive some doses via one route and other doses via another route. The administration type with id ‘3’ is not used in the model, thus all doses with this id will not be applied.
The default value for administration types is 1. This means that if there is no column ADMINISTRATION ID in the dataset, all doses will be associated to the administration id 1. If the dataset contains a column ADMINISTRATION ID but the structural model does not include arguments adm to indicate how to apply the different types of doses, only the doses with administration id ‘1’ in the data set will be used.
Changes compared to the 2016R1 version: In the 2016R1 version the ADM column could be used simultaneously as administration identifier for dose-lines and as observation identifier for response-lines. In the 2018R1 version the ADMINISTRATION ID column is used only for dose lines.
Format restrictions:
- For dose-lines, the column shall contain only positive integers. For response-lines, dots ‘.’ or integers are allowed.
- A data set shall not contain more than one ADMINISTRATION ID column-type.
INFUSION RATE and INFUSION DURATION (formerly RATE and TINF): rate and duration of infusion
These columns enable to define the rate (INFUSION RATE column-type) or duration (INFUSION DURATION column-type) of doses administered as infusions. The column content is meaningful only for dose-lines. The rate and duration information is transferred to the model via the use of the iv macro, depot macro or pkmodel macro. If a RATE is defined, the duration of the infusion will be AMOUNT/RATE. If a DURATION is defined, the rate will be AMOUNT/DURATION. The units must be coherent with the units of the AMOUNT and TIME columns. A dose-lines, if a negative value, or dot ‘.’ or 0 is used, the infusion will be replaced by a bolus.
We strongly recommend to have small duration values (less than 10). Indeed, if the duration is too long, the calculation of the analytical solution of the model may produce NaN. Two workarounds are possible:
– Either rescale the time units to have smaller duration values.
– Use ODEs instead of analytical solutions. ODEs can be enforced with useAnalyticalSolution = no in the model file.
Examples:
- Basic example: with the following data set, assuming the TIME units are minutes and AMOUNT units mg, an amount of 10 mg will be applied via an infusion over 5 minutes starting at time 0. This is equivalent to a RATE of 2 mg/minute. The 20 mg doses at time 2, 3, and 4 minutes are applied via a bolus.
ID TIME AMT TINF Y 1 0 10 5 . 1 1 . . 6 1 2 20 0 . 1 3 20 -2 . 1 4 20 . .
Format restrictions:
- A data set shall not contain more than one column with column-type INFUSION RATE or INFUSION DURATION.
- A negative value, “.” or 0 means a bolus dose, without any infusion rate or time.
- For dose-lines, the value must be a double, or a dot ‘.’.
- For response lines, the content is free.
STEADY STATE and INTERDOSE INTERVAL (formerly SS and II): steady-state and inter-dose interval
Steady-state is used to specify that any transitory effect is over and that the system response is now a periodic function of doses. In practice, a fixed number of doses (by default 5) is added before the dose entered with the STEADY STATE flag (so 6 doses in total, by default). The period between doses is set to the INTERDOSE INTERVAL. The number of doses to add can be changed when loading the data and will be saved in the project file. The number of added doses may be reduced compared to the specified value if the doses to add would interfere with another dose or observation: any steady-state dose that would be added before (i.e earlier in time) a dose or response line is not added, even if the line information is ignored (for instance with EVENT ID = 2 or IGNORED OBSERVATION = 1).
The allowed values are the following:
- STEADY-STATE = 0 or ‘.’ : no steady-state, the dose is a normal dose.
- STEADY-STATE = 1 : the system has achieved steady-state. A washout (i.e reset to the initial values of the model) is applied before the first added dose.
- STEADY-STATE = 2 or 3 : the system has achieved steady-state. Unlike STEADY-STATE = 1, no washout is added.
STEADY-STATE and EVENT ID: when STEADY-STATE = 1 is used on the same line as EVENT ID = 3 or 4, a washout is applied just before the first dose added for steady-state (because of STEADY-STATE = 1) and also just before the dose defined on that line (because of EVENT ID = 3 or 4). When STEADY-STATE = 2 or 3 is used on the same line as EVENT ID = 3 or 4, a washout is added just before the dose defined on that line.
STEADY-STATE and REGRESSOR: see the REGRESSOR page.
Changes with respect to the MonolixSuite2016R1 version:
- In versions prior MonolixSuite2018R1, the number of added doses can be changed in the preferences.xmlx file, located in
<home>/lixoft/monolix/configin the user folder. By default, the number of doses is 5 and was defined in the line<dosesToAddForSteadyState value="5"/>.It can for instance be changed to<dosesToAddForSteadyState value="20"/>. - In versions prior MonolixSuite2018R1, SS=1 was generating new occasions. This is not the case anymore.
- In versions prior MonolixSuite2018R1, SS=2 and SS=3 were not allowed.
Examples:
- Basic example: On the following example:
ID TIME AMT SS II Y 1 0 10 1 2 .
5 doses are applied, at times -10, -8, -6, -4, -2 in addition to the dose at time = 0. The above data set is thus equivalent to:
ID TIME AMT SS II Y 1 -10 10 0 0 . 1 -8 10 0 0 . 1 -6 10 0 0 . 1 -4 10 0 0 . 1 -2 10 0 0 . 1 0 10 0 0 .
The first added dose will have a wash-out.
- Example with informations colliding with doses to add: The following data set, with a normal dose at t=0 and a steady-state dose at t=10 with an interdose-interval of 3.5 will lead to the following situation:
ID TIME Y AMT SS II 1 0 . 10 0 0 1 0 10 . . . 1 1 6 . . . 1 2 4 . . . 1 10 . 10 1 3.5 1 11 9 . . . 1 12 6 . . . 1 13 3 . . . 1 14 2 . . . |
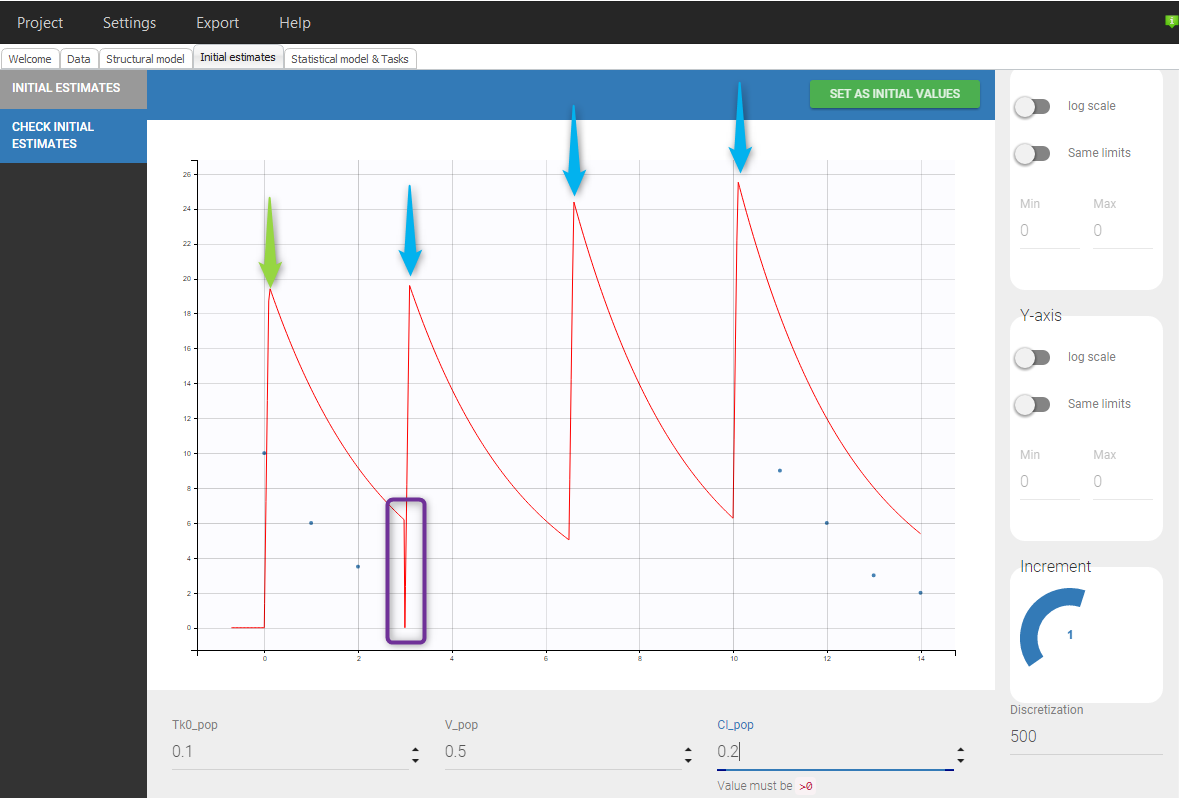 |
Even if 5 additional doses are specified in the GUI when loading the data, there will be only 2 additional doses as otherwise the previous measurements would be interfered with. In addition, there is a washout (highlighted in purple) just before the first added dose.If we would replace SS=1 by SS=2 on line 5, we would again have only 2 doses added but no wash out as can be seen in the following figure:
ID TIME Y AMT SS II 1 0 . 10 0 0 1 0 10 . . . 1 1 6 . . . 1 2 4 . . . 1 10 . 10 2 3.5 1 11 9 . . . 1 12 6 . . . 1 13 3 . . . 1 14 2 . . . |
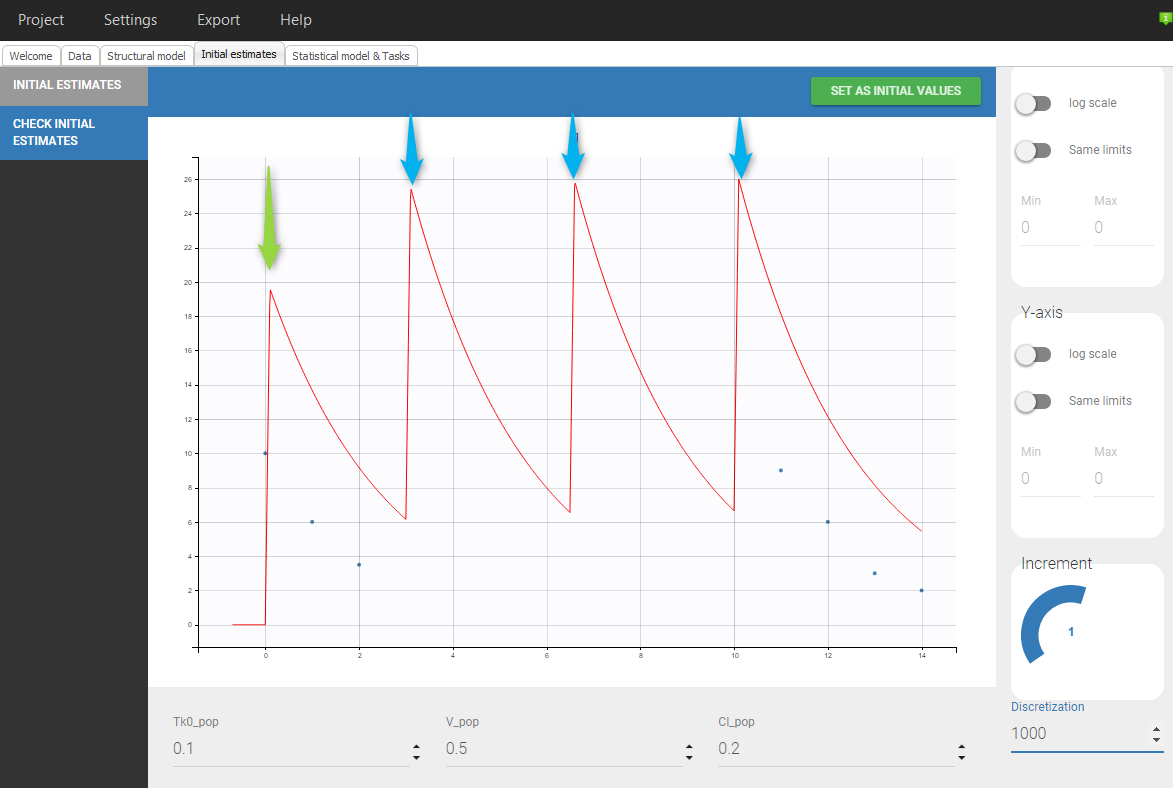 |
- Typical example with pre-dose PK measurement, dose at site and post-dose PK measurements: the subject takes regularly doses at home. During a hospital visit, he gets a pre-dose PK measurement, a dose at site, and one or several post-dose PK measurements. The STEADY-STATE statement cannot be applied on the dose administered at site, as the doses to add would interfere with the pre-dose PK measurement. We thus need to write:
ID TIME AMT SS II Y 1 0 10 1 24 . ; steady-state statement 1 23.9 . . . 5.5 ; pre-dose measurement 1 24 10 . . . ; dose at site 1 25 . . . 9.9 ; post-dose measurement
FAQ:
- Can I define two different STEADY-STATE lines for morning and evening doses? No, as the doses to add would collide with each other. You will have to use an ADDITIONAL DOSES column instead.
Format restrictions for STEADY-STATE:
- A data set shall not contain more than one column with column-type STEADY STATE.
- If there is a column-type STEADY STATE, there should be a column-type INTERDOSE INTERVAL.
- Accepted values (for all lines, including response-lines): 0, 1, 2, 3 or dot ‘.’
Format restrictions for INTERDOSE-INTERVAL:
- A data set shall not contain more than one column with column-type INTERDOSE INTERVAL.
- If there is a column-type STEADY STATE, there should be a column-type INTERDOSE INTERVAL.
- Accepted values:
- on response-line: 0 or ‘.’ (non-zero double value will generate a warning)
- on normal dose-line (no STEADY-STATE or ADDITIONAL DOSE): 0 or ‘.’ (non-zero double value will generate a warning)
- on STEADY-STATE or ADDITIONAL DOSE line: non-zero double value
- Clock format is not accepted
ADDITIONAL DOSES (formerly ADDL): additional dose line
The ADDITIONAL DOSES column is a useful shortcut to specify dose regimens with repetitive treatments. The value in ADDITIONAL DOSES is the number of times the dose shall be repeated (in additional to the dose defined on that line) and column INTERDOSE INTERVAL contains the dose repetition interval. Doses that would be added after the last measurement are useless and will thus not be added.
ADDITIONAL DOSES and REGRESSOR: see the REGRESSOR page.
Examples:
- Basic example: For instance to specify a dose of 10 every 12 hours during 3 days it is possible to write:
ID TIME AMT Tom 0 10 Tom 12 10 Tom 24 10 Tom 36 10 Tom 48 10 Tom 60 10 Tom 72 10
but ADDITIONAL DOSES (ADDL) and INTERDOSE INTERVAL (II) can also be used to specify the same information in a single line
ID TIME AMT ADDL II Tom 0 10 6 12
Format restrictions:
- When there is an ADDL column there must be an INTERDOSE INTERVAL (interdose interval) column to indicate the inter dose timing.
- For dose-lines with ADDL strictly positive, the INTERDOSE INTERVAL value must be strictly positive.
- Accepted values:
- on normal dose lines: 0 or ‘.’
- on response lines: 0 or ‘.’
- on lines where ADDITIONAL DOSES is needed: positive integer